Effects of Heat Treatment on Fluorescence Properties of Humic Substances from Sandy Soil in Arid Land and Their Hg(II) Binding Behaviors
2011-12-20
Mercury (Hg) is a highly mobile heavy metal, and the soil can act as a sink or source in the geochemical/biogeochemical cycle of Hg. The fate and transport of Hg are strongly influenced by its interaction with organic ligands in the aquatic and terrestrial environments. The organic matters are recognized as the most important soil component controlling Hg retention behavior in soils.
Humic substances, heterogeneous mixtures of a variety of organic compounds, are ubiquitous in water, sediments and soils. Humic substances have a strong ability to bind Hg(II) due to their high contents of oxygen-containing functional groups, such as carboxyl, hydroxyl, and carbonyl. Therefore, they may affect the concentration, chemical forms and bioavailability of Hg in the environment.
The topsoil temperature in arid areas of Xinjiang, China can be up to about 80oC in summer. This may significantly affect the chemical properties of soil humic substances. However, the effects of high temperature on characteristics of soil humic substances and their complexation with toxic metals are still poorly known. In the present study, binding of Hg(II) to unheated soil humic substances and heated soil humic substances from sandy soils was comparatively investigated using three-dimensional excitation–emission matrix (EEM) fluorescence spectroscopy.
Therefore, researchers used EEM fluorescence spectroscopy to examine the effects of high temperature on the fluorescence feature of humic substances from sandy soil and their binding ability to Hg(II). Two fluorescent peaks (peak I at Ex/Em = 365–370/470–474 nm; peak II at Ex/Em = 270–275/468–472 nm) identified as humic-like fluorescence were observed in the EEM spectra of humic substances. Both peaks were clearly quenched by Hg(II), indicating the strong interaction of humic-like substances with Hg(II), and showed blue shifts after heat treatment. Heat treatment caused an increase of the fraction of accessible fluorophore (fa), binding sites number (n) and effective quenching constants (logKa), indicating that more binding sites in humic substances could bind Hg(II) and form more stable humic substances–Hg(II) complexes after heat treatment. However, a decrease of binding constants (logKb) suggested that heat treatment would reduce the binding capacity of each binding site of humic substances to Hg(II). This study implies the transport of Hg(II) may be affected by high temperature in the arid zone due to the modification of the physicochemical properties of humic substances in soil.
This work has been published on Environmental Earth Sciences, 2011 (http://www.springerlink.com/content/32027502626x782t/).
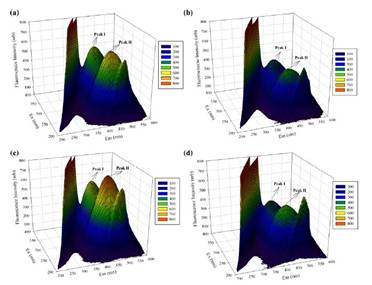
Three-dimensional EEM plots of humic substances solutions: a UHS unheated soil humic substances, b UHS + 133 μM Hg(II), c HHS heated soil humic substances, d HHS + 133μM Hg(II)



